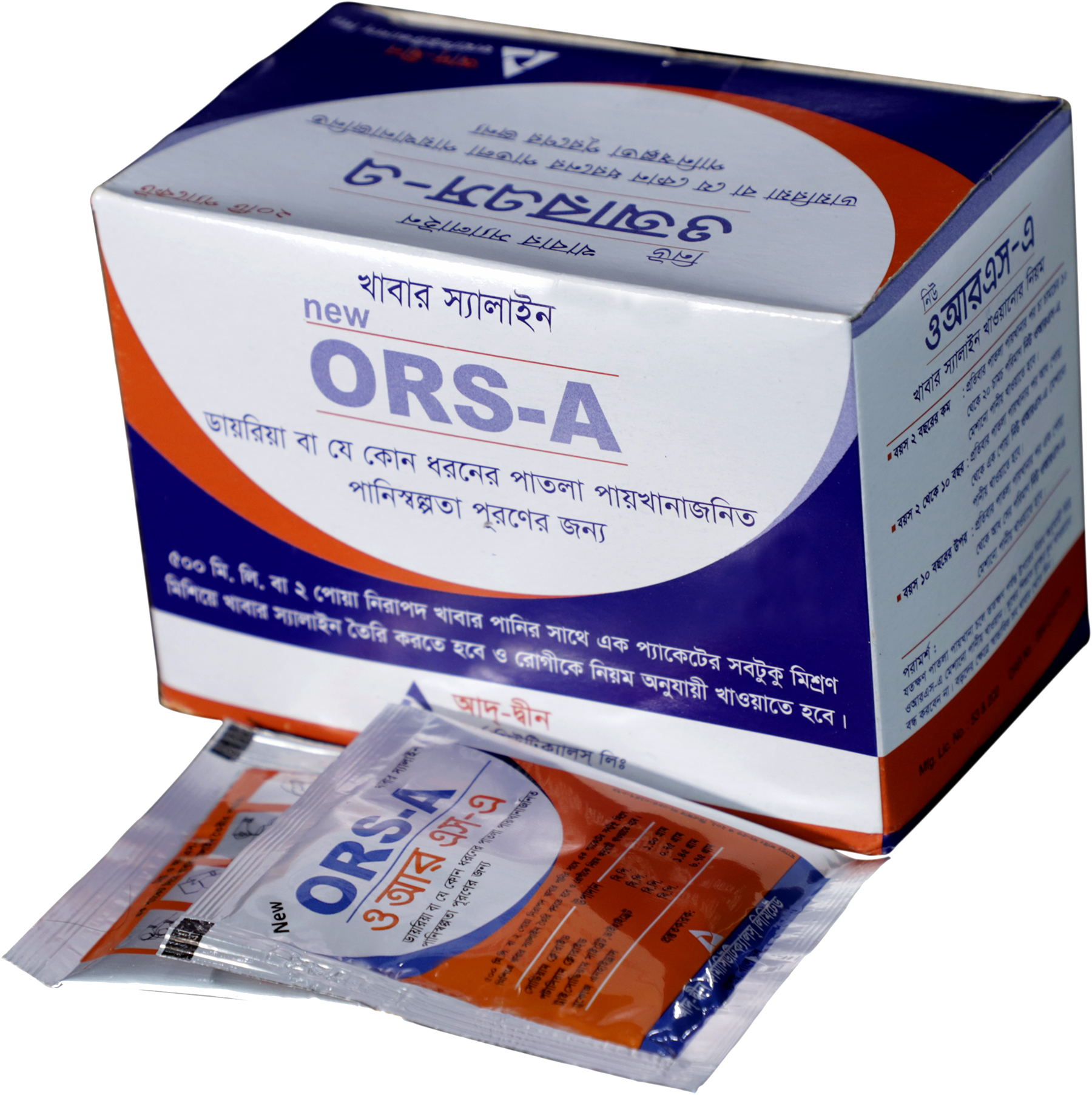
COMPOSITION
1/2 liter (New ORS-A) formula (10.25 gm): Per sachet contains-
- Sodium chloride 1.30gm
- Potassium chloride 0.75 gm
- Tri-sodium citrate dihydrate 1.45 gm
- Glucose anhydrous 6.75 gm
PHARMACOLOGY
Oral rehydration salts are given orally to prevent or treat dehydration due to acute diarrhoea. Essential water and salts are lost in stools and vomiting, and dehydration results when blood volume is decreased because of fluid loss from the extracellular fluid compartment. Preservation of the facilitated glucose-sodium co-transport system in the small-bowel mucosa is the rationale of oral rehydration therapy. Glucose is actively absorbed in the normal intestine and carries sodium with it in about an equimolar ration. Therefore, there is a greater net absorption of an isotonic salt solution with glucose than one without it.
Potassium replacement during acute diarrhoea prevents below-normal serum concentrations of potassium, especially in children, in whom stool potassium losses are higher than in adults. Bicarbonates are effective in correcting the metabolic acidosis caused by diarrhoea and dehydration.
INDICATION
Oral Rehydration Salt (New ORS-A) is indicated in Diarrhoea (treatment) and Electrolyte depletion (prophylaxis and treatment)
DOSAGE AND ADMINISTRATION
- Children less than 2 years: After each loose stool or vomiting 10 to 20 spoonful (50-100 ml).
- Children 2 to 10 years: After each loose stool or vomiting 100-200 ml of prepared oral saline.
- Adult and children above 10 years: After each loose stool or vomiting 200-400 ml of prepared saline.
CONTRAINDICATION
No known contraindications
WARNING AND PRECAUTION
Depressed renal function, severe continuing diarrhoea or other critical fluid losses may need supplementation with parenteral fluids along with oral saline. Solutions containing acetate or gluconate ions should be used with caution, as excess administration may result in metabolic alkalosis. Solutions containing dextrose should be used with caution in patients with known subclinical or overt diabetes mellitus.
SIDE EFFECTS
No known side effects
USE IN PREGNANCY AND LACTATION
FDA has not yet classified the drug into a specified pregnancy category
DRUG INTERACTION
There are no known drug interactions and none well documented.
STORAGE
Store at a cool & dry place, protect from light & moisture. Keep out of the reach of children.
PACKING
New ORS-A: Box containing 20 X 1’s sachet in ALu ALu pack