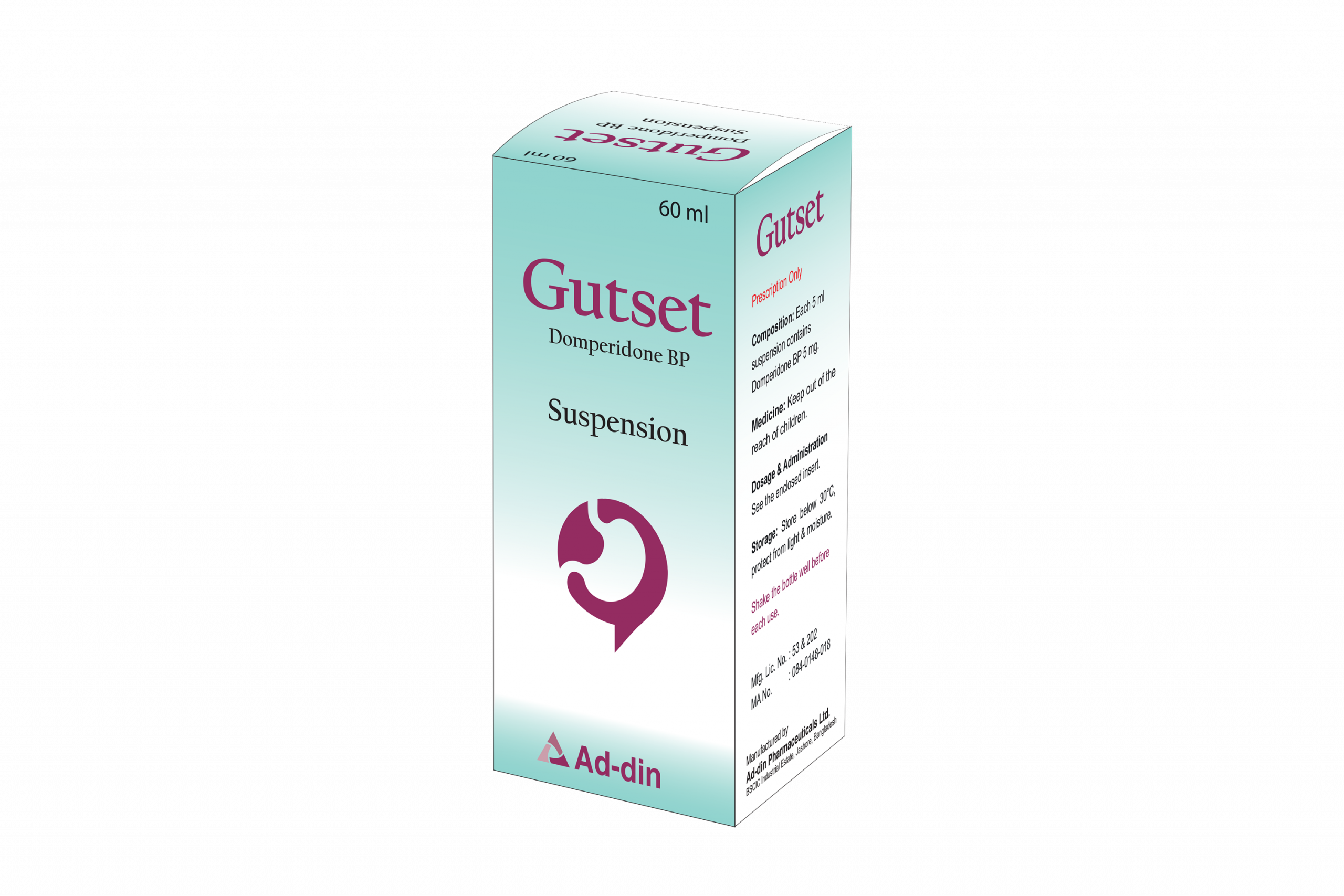
Composition
Each 5 ml suspension contains Domperidone BP 5 mg.
Pharmacology
Gutset is a dopamine antagonist that principally blocks the dopamine receptors located in the Chemoreceptor Trigger Zone (CTZ) and stomach. Its gastroprokinetic action is based on its blocking effect of dopamine receptors that have an influence on the motility of gastro-intestinal tract. Due to its weak penetration across the blood-brain barrier, Gutset has almost no effect on the dopaminergic receptors in the brain therefore excluding psychotropic and neurologic side effects. Gutset restores normal motility and tone of the upper gastro-intestinal tract, facilitates gastric emptying, enhances antral and duodenal peristalsis and regulates contraction of the pylorus. Gutset also increases esophageal peristalsis and lower esophageal sphincter pressure, and thus prevents regurgitation of gastric content.
Indications
1. Stimulation of gut mobility
a) Non-ulcer dyspepsia
b) Esophageal reflux, reflux esophagitis and gastritis
c) Diabetic gastroparesis
d) Functional dyspepsia
e) Speeding barium transit in ‘follow-through’ radiological studies
2. Prevention and symptomatic relief of acute nausea and vomiting from any cause including cytotoxic therapy, radio therapy and anti-parkinsonism therapy
3. In the treatment of migraine.
Dosage & Administration:
The recommended oral dose for
Adults: 10 – 20 mg every 4 – 8 hours daily
Children: 0.2 – 0.4 mg/kg every 4 – 8 hours daily.
Note: Gutset tablet and suspension should be taken 15 – 30 minutes before a meal.
For acute nausea and vomiting, maximum period of treatment is 12 weeks.
Contraindications
Domperidone is contraindicated to patients having known hypersensitivity to this drug and in case of neonates. Domperidone should not be used whenever gastro-intestinal stimulation might be dangerous i.e., gastro-intestinal hemorrhage, mechanical obstruction or perforation. Also contraindicated in patients with prolactin releasing pituitary tumor (prolactinoma).
Side Effects
Domperidone may produce hyperprolactinemia(1.3% frequency). This may result in galactorrhea, breast enlargement and soreness and reduced libido. Dry mouth (1.9%), thirst, headache (1.2%), nervousness, drowsiness (0.4%), diarrhea (0.2%), skin rash and itching (0.1%) may occur during treatment with Domperidome. Extra-pyramidal reactions are seen in 0.05% of patients in clinical studies.
Precaution
Domperidone should be used with absolute caution in case of children because there may be increased risk of extra-pyramidal reactions in young children because of an incompletely developed blood-brain barrier. Since domperidone is highly metabolized in liver, it should be used with caution in patient with hepatic impairment.
Use in Pregnancy & Lactation
Pregnant women: The safety of Domperidone has not been proven and it is therefore not recommended during pregnancy. Animal studies have not demonstrated teratogenic effects on the fetus.
Lactating mother: Domperidone may precipitate galactorrhea and improve post-natal lactation. It is secreted in breast milk but in very small quantities insufficient to be considered harmful.
Drug Interactions
Domperidone may reduce the risk of hypoprolactemic effect of bromocriptine. The action of Domperidone on GI function may be antagonized by anti-muscarinics and opoid analgesics. Care should be exercised when domperidone is administered in combination with MAO (monoamine oxidase) inhibitors.
Storage
Store below 30ºC, Protected from light & moisture. Keep out of children’s reach.
Packaging
Each bottle contains 60 ml of suspension.